Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disorder that may affect many tissues and organs, but principally attacks synovial joints. The process produces an inflammatory response of the synovium (synovitis) secondary to hyperplasia of synovial cells, excess synovial fluid, and the development of pannus in the synovium. The pathology of the disease process often leads to the destruction of articular cartilage and ankylosis of the joints. Rheumatoid arthritis can also produce diffuse inflammation in the lungs, pericardium, pleura, and sclera, and also nodular lesions, most common in subcutaneous tissue. Although the cause of rheumatoid arthritis is unknown, autoimmunity plays a pivotal role in both its chronicity and progression, and RA is considered a systemic autoimmune disease.
About 1% of the world's population is afflicted by rheumatoid arthritis, women three times more often than men. Onset is most frequent between the ages of 40 and 50, but people of any age can be affected. It can be a disabling and painful condition, which can lead to substantial loss of functioning and mobility if not adequately treated. It is a clinical diagnosis made on the basis of symptoms, physical exam, radiographs (X-rays) and labs, although the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) publish diagnostic guidelines. Diagnosis and long-term management are typically performed by a rheumatologist, an expert in joint, muscle and bone diseases.[1]
Various treatments are available. Non-pharmacological treatment includes physical therapy, orthoses, occupational therapy and nutritional therapy but do not stop progression of joint destruction. Analgesia (painkillers) and anti-inflammatory drugs, including steroids, are used to suppress the symptoms, while disease-modifying antirheumatic drugs (DMARDs) are required to inhibit or halt the underlying immune process and prevent long-term damage. In recent times, the newer group of biologics has increased treatment options.[1]
The name is based on the term "rheumatic fever", an illness which includes joint pain and is derived from the Greek word ῥεύμα-rheuma (nom.), ῥεύματος-rheumatos (gen.) ("flow, current"). The suffix -oid ("resembling") gives the translation as joint inflammation that resembles rheumatic fever. The first recognized description of rheumatoid arthritis was made in 1800 by Dr. Augustin Jacob Landré-Beauvais (1772–1840) of Paris.[2]
[edit] Signs and symptoms
While rheumatoid arthritis primarily affects joints, problems involving other organs of the body are known to occur. Extra-articular ("outside the joints") manifestations other than anemia (which is very common) are clinically evident in about 15–25% of individuals with rheumatoid arthritis.[3] It can be difficult to determine whether disease manifestations are directly caused by the rheumatoid process itself, or from side effects of the medications commonly used to treat it – for example, lung fibrosis from methotrexate or osteoporosis from corticosteroids.
[edit] Joints
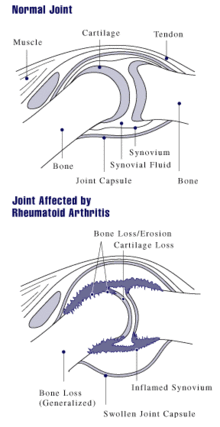
A diagram showing how rheumatoid arthritis affects a joint
The arthritis of joints known as synovitis is inflammation of the synovial membrane that lines joints and tendon sheaths. Joints become swollen, tender and warm, and stiffness limits their movement. With time RA nearly always affects multiple joints (it is a polyarthritis), most commonly small joints of the hands, feet and cervical spine, but larger joints like the shoulder and knee can also be involved. Synovitis can lead to tethering of tissue with loss of movement and erosion of the joint surface causing deformity and loss of function.[1]
Rheumatoid arthritis typically manifests with signs of inflammation, with the affected joints being swollen, warm, painful and stiff, particularly early in the morning on waking or following prolonged inactivity. Increased stiffness early in the morning is often a prominent feature of the disease and typically lasts for more than an hour. Gentle movements may relieve symptoms in early stages of the disease. These signs help distinguish rheumatoid from non-inflammatory problems of the joints, often referred to as osteoarthritis or "wear-and-tear" arthritis. In arthritis of non-inflammatory causes, signs of inflammation and early morning stiffness are less prominent with stiffness typically less than 1 hour, and movements induce pain caused by mechanical arthritis.[4] In RA, the joints are often affected in a fairly symmetrical fashion, although this is not specific, and the initial presentation may be asymmetrical.
As the pathology progresses the inflammatory activity leads to tendon tethering and erosion and destruction of the joint surface, which impairs range of movement and leads to deformity. The fingers may suffer from almost any deformity depending on which joints are most involved. Medical students are taught to learn names for specific deformities, such as ulnar deviation, boutonniere deformity, swan neck deformity and "Z-thumb," but these are of no more significance to diagnosis or disability than other variants, since they occur in osteoarthritis as well. "Z-thumb" or "Z-deformity" consists of hyperextension of the interphalangeal joint, fixed flexion and subluxation of the metacarpophalangeal joint and gives a "Z" appearance to the thumb.
The rheumatoid nodule, which is often subcutaneous, is the cutaneous feature most characteristic of rheumatoid arthritis. The initial pathologic process in nodule formation is unknown but may be essentially the same as the synovitis, since similar structural features occur in both. The nodule has a central area of fibrinoid necrosis that may be fissured and which corresponds to the fibrin-rich necrotic material found in and around an affected synovial space. Surrounding the necrosis is a layer of palisading macrophages and fibroblasts, corresponding to the intimal layer in synovium and a cuff of connective tissue containing clusters of lymphocytes and plasma cells, corresponding to the subintimal zone in synovitis. The typical rheumatoid nodule may be a few millimetres to a few centimetres in diameter and is usually found over bony prominences, such as the olecranon, the calcaneal tuberosity, the metacarpophalangeal joint, or other areas that sustain repeated mechanical stress. Nodules are associated with a positive RF (rheumatoid factor) titer and severe erosive arthritis. Rarely, these can occur in internal organs or at diverse sites on the body.
Several forms of vasculitis occur in rheumatoid arthritis. A benign form occurs as microinfarcts around the nailfolds. More severe forms include livedo reticularis, which is a network (reticulum) of erythematous to purplish discoloration of the skin caused by the presence of an obliterative cutaneous capillaropathy.
Other, rather rare, skin associated symptoms include:
Fibrosis of the lungs is a recognized response to rheumatoid disease. It is also a rare but well recognized consequence of therapy (for example with methotrexate and leflunomide). Caplan's syndrome describes lung nodules in individuals with rheumatoid arthritis and additional exposure to coal dust. Pleural effusions are also associated with rheumatoid arthritis. Another complication of RA is Rheumatoid Lung Disease. It is estimated that about one quarter of Americans with RA develop Rheumatoid Lung Disease.[5]
[edit] Kidneys
Renal amyloidosis can occur as a consequence of chronic inflammation.[6] Rheumatoid arthritis may affect the kidney glomerulus directly through a vasculopathy or a mesangial infiltrate but this is less well documented (though this is not surprising, considering immune complex-mediated hypersensitivities are known for pathogenic deposition of immune complexes in organs where blood is filtered at high pressure to form other fluids, such as urine and synovial fluid[7]). Treatment with Penicillamine and gold salts are recognized causes of membranous nephropathy.
[edit] Heart and blood vessels
People with rheumatoid arthritis are more prone to atherosclerosis, and risk of myocardial infarction (heart attack) and stroke is markedly increased.[8][9] Other possible complications that may arise include: pericarditis, endocarditis, left ventricular failure, valvulitis and fibrosis.[10] Many people with rheumatoid arthritis do not experience the same chest pain that others feel when they have angina or myocardial infarction. To reduce cardiovascular risk, it is crucial to maintain optimal control of the inflammation caused by rheumatoid arthritis (which may be involved in causing the cardiovascular risk), and to use exercise and medications appropriately to reduce other cardiovascular risk factors such as blood lipids and blood pressure. Doctors who treat rheumatoid arthritis patients should be sensitive to cardiovascular risk when prescribing anti-inflammatory medications, and may want to consider prescribing routine use of low doses of aspirin if the gastrointestinal effects are tolerable.[10]
-
Ocular
-
The eye is directly affected in the form of episcleritis which when severe can very rarely progress to perforating scleromalacia. Rather more common is the indirect effect of keratoconjunctivitis sicca, which is a dryness of eyes and mouth caused by lymphocyte infiltration of lacrimal and salivary glands. When severe, dryness of the cornea can lead to keratitis and loss of vision. Preventive treatment of severe dryness with measures such as nasolacrimal duct occlusion is important.
-
Hepatic
-
Cytokine production in joints and/or hepatic Kupffer cells leads to increased activity of hepatocytes with increased production of acute-phase proteins, such as C-reactive protein, and increased release of enzymes such as alkaline phosphatase into the blood. In Felty's syndrome, Kuppfer cell activation is so marked that the resulting increase in hepatocyte activity is associated with nodular hyperplasia of the liver, which may be palpably enlarged. Although Kupffer cells are within the hepatic parenchyma, they are separate from hepatocytes. As a result there is little or no microscopic evidence of hepatitis (immune-mediated destruction of hepatocytes). Hepatic involvement in RA is essentially asymptomatic.
-
Hematological
-
Anemia is by far the most common abnormality of the blood cells. Rheumatoid arthritis may cause a warm autoimmune hemolytic anemia.[11] The red cells are of normal size and colour (normocytic and normochromic). A low white blood cell count (neutropenia) usually only occurs in patients with Felty's syndrome with an enlarged liver and spleen. The mechanism of neutropenia is complex. An increased platelet count (thrombocytosis) occurs when inflammation is uncontrolled, as does the anemia.
-
Neurological
-
Peripheral neuropathy and mononeuritis multiplex may occur. The most common problem is carpal tunnel syndrome caused by compression of the median nerve by swelling around the wrist. Atlanto-axial subluxation can occur, owing to erosion of the odontoid process and or/transverse ligaments in the cervical spine's connection to the skull. Such an erosion (>3mm) can give rise to vertebrae slipping over one another and compressing the spinal cord. Clumsiness is initially experienced, but without due care this can progress to quadriplegia.
-
Constitutional symptoms
-
Constitutional symptoms including fatigue, low grade fever, malaise, morning stiffness, loss of appetite and loss of weight are common systemic manifestations seen in patients with active rheumatoid arthritis.
-
Osteoporosis
-
Local osteoporosis occurs in RA around inflamed joints. It is postulated to be partially caused by inflammatory cytokines. More general osteoporosis is probably contributed to by immobility, systemic cytokine effects, local cytokine release in bone marrow and corticosteroid therapy.
-
Lymphoma
-
The incidence of lymphoma is increased in RA, although it is still uncommon.[12][13]
[edit] Diagnosis
[edit] Imaging

X-ray of the hand in rheumatoid arthritis.
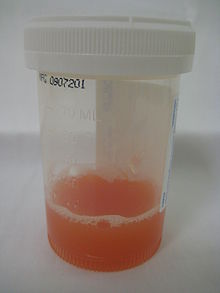
Appearance of synovial fluid from a joint with inflammatory arthritis.

Signs of destruction and inflammation on
ultrasonography and
magnetic resonance imaging in the second
metacarpophalangeal joint in established rheumatoid arthritis. Thin arrows indicate an erosive change; thick arrows indicate
synovitis. Ultrasonography (left side of image) in the (a) longitudinal and (b) the transverse planes shows both signs of destruction and inflammation. Axial T1-weighted magnetic resonance images were obtained (c) before and (d) after contrast administration, also demonstrating synovitis. Additionally, a coronal T1-weighted magnetic resonance image (e) before contrast administration visualizes the same bone erosion as shown in panels c and d.
X-rays of the hands and feet are generally performed in people with a polyarthritis. In rheumatoid arthritis, there may be no changes in the early stages of the disease, or the x-ray may demonstrate juxta-articular osteopenia, soft tissue swelling and loss of joint space. As the disease advances, there may be bony erosions and subluxation. X-rays of other joints may be taken if symptoms of pain or swelling occur in those joints.
Other medical imaging techniques such as magnetic resonance imaging (MRI) and ultrasound are also used in rheumatoid arthritis.[citation needed]
There have been technical advances in ultrasonography. High-frequency transducers (10 MHz or higher) have improved the spatial resolution of ultrasound images; these images can depict 20% more erosions than conventional radiography. Also, color Doppler and power Doppler ultrasound, which show vascular signals of active synovitis depending on the degree of inflammation, are useful in assessing synovial inflammation. This is important, since in the early stages of rheumatoid arthritis, the synovium is primarily affected, and synovitis seems to be the best predictive marker of future joint damage.[14]
[edit] Blood tests
When RA is clinically suspected, immunological studies are required, such as testing for the presence of rheumatoid factor (RF, a non-specific antibody).[15] A negative RF does not rule out RA; rather, the arthritis is called seronegative. This is the case in about 15% of patients.[16] During the first year of illness, rheumatoid factor is more likely to be negative with some individuals converting to seropositive status over time. RF is also seen in other illnesses, for example Sjögren's syndrome, Hepatitis C, chronic infections and in approximately 10% of the healthy population, therefore the test is not very specific.
Because of this low specificity, new serological tests have been developed, which test for the presence of the anti-citrullinated protein antibodies (ACPAs) or anti-CCP. Like RF, these tests are positive in only a proportion (67%) of all RA cases, but are rarely positive if RA is not present, giving it a specificity of around 95%.[16] As with RF, there is evidence for ACPAs being present in many cases even before onset of clinical disease.[citation needed]
The most common tests for ACPAs are the anti-CCP (cyclic citrullinated peptide) test and the Anti-MCV assay (antibodies against mutated citrullinated Vimentin). Recently a serological point-of-care test (POCT) for the early detection of RA has been developed. This assay combines the detection of rheumatoid factor and anti-MCV for diagnosis of rheumatoid arthritis and shows a sensitivity of 72% and specificity of 99.7%.[17][18]
Also, several other blood tests are usually done to allow for other causes of arthritis, such as lupus erythematosus. The erythrocyte sedimentation rate (ESR), C-reactive protein, full blood count, renal function, liver enzymes and other immunological tests (e.g., antinuclear antibody/ANA) are all performed at this stage. Elevated ferritin levels can reveal hemochromatosis, a mimic RA, or be a sign of Still's disease [disambiguation needed], a seronegative, usually juvenile, variant of rheumatoid.[citation needed]
[edit] Criteria
In 2010 the 2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria were introduced.[19] These new classification criteria overruled the "old" ACR criteria of 1987 and are adapted for early RA diagnosis. The "new" classification criteria, jointly published by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) establish a point value between 0 and 10. Every patient with a point total of 6 or higher is unequivocally classified as an RA patient, provided he has synovitis in at least one joint and given that there is no other diagnosis better explaining the synovitis. Four areas are covered in the diagnosis:[19]
-
joint involvement, designating the metacarpophalangeal joints, proximal interphalangeal joints, the interphalangeal joint of the thumb, second through third metatarsophalangeal joint and wrist as small joints, and elbows, hip joints and knees as large joints:
-
Involvement of 1 large joint gives 0 points
-
Involvement of 2-10 large joints gives 1 point
-
Involvement of 1-3 small joints (with or without involvement of large joints) gives 2 points
-
Involvement of4-10 small joints (with or without involvement of large joints) gives 3 points
-
Involvement of more than 10 joints (with involvement of at least 1 small joint) gives 5 points
-
serological parameters – including the rheumatoid factor as well as ACPA – "ACPA" stands for "anti-citrullinated protein antibody":
-
Negative RF and negative ACPA gives 0 points
-
Low-positive RF or low-positive ACPA gives 2 points
-
High-positive RF or high-positive ACPA gives 3 points
-
acute phase reactants: 1 point for elevated erythrocyte sedimentation rate, ESR, or elevated CRP value (c-reactive protein)
-
duration of arthritis: 1 point for symptoms lasting six weeks or longer
The new criteria accommodate to the growing understanding of rheumatoid arthritis and the improvements in diagnosing RA and disease treatment. In the "new" criteria serology and autoimmune diagnostics carries major weight, as ACPA detection is appropriate to diagnose the disease in an early state, before joints destructions occur. Destruction of the joints viewed in radiological images was a significant point of the ACR criteria from 1987.[20] This criterion no longer is regarded to be relevant, as this is just the type of damage that treatment is meant to avoid.
The criteria are not intended for the diagnosis for routine clinical care; they were primarily intended to categorize research (classification criteria). In clinical practice, the following criteria apply:[citation needed]
-
two or more swollen joints
-
morning stiffness lasting more than one hour for at least six weeks
-
the detection of rheumatoid factors or autoantibodies against ACPA such as autoantibodies to mutated citrullinated vimentin can confirm the suspicion of rheumatoid arthritis. A negative autoantibody result does not exclude a diagnosis of RA.
[edit] Differential diagnoses
Several other medical conditions can resemble RA, and usually need to be distinguished from it at the time of diagnosis:[21][22]
-
Crystal induced arthritis (gout, and pseudogout) – usually involves particular joints (knee, MTP1, heels) and can be distinguished with aspiration of joint fluid if in doubt. Redness (RA doesn't have redness at the joints), asymmetric distibution of affected joints, pain occurs at night and the starting pain is less than an hour with gout.
-
Osteoarthritis – distinguished with X-rays of the affected joints and blood tests, age (mostly older patients), starting pain less than an hour, a-symetric distribution of affected joints and pain worsens when using joint for longer periods.
-
Systemic lupus erythematosus (SLE) – distinguished by specific clinical symptoms and blood tests (antibodies against double-stranded DNA)
-
One of the several types of psoriatic arthritis resembles RA – nail changes and skin symptoms distinguish between them
-
Lyme disease causes erosive arthritis and may closely resemble RA – it may be distinguished by blood test in endemic areas
-
Reactive arthritis (previously Reiter's disease) – asymmetrically involves heel, sacroiliac joints, and large joints of the leg. It is usually associated with urethritis, conjunctivitis, iritis, painless buccal ulcers, and keratoderma blennorrhagica.
-
Ankylosing spondylitis – this involves the spine and is usually diagnosed in males, although a RA-like symmetrical small-joint polyarthritis may occur in the context of this condition.
-
Hepatitis C – RA-like symmetrical small-joint polyarthritis may occur in the context of this condition. Hepatitis C may also induce Rheumatoid Factor auto-antibodies
Rarer causes that usually behave differently but may cause joint pains:[21]
-
Sarcoidosis, amyloidosis, and Whipple's disease can also resemble RA.
-
Hemochromatosis may cause hand joint arthritis.
-
Acute rheumatic fever can be differentiated from RA by a migratory pattern of joint involvement and evidence of antecedent streptococcal infection. Bacterial arthritis (such as streptococcus) is usually asymmetric, while RA usually involves both sides of the body symmetrically.
-
Gonococcal arthritis (another bacterial arthritis) is also initially migratory and can involve tendons around the wrists and ankles.
[edit] Monitoring progression
The progression of rheumatoid arthritis can be followed using scores such as Disease Activity Score of 28 joints (DAS28). It is widely used as an indicator of RA disease activity and response to treatment, but is not always a reliable indicator of treatment effect.[23] The joints included in DAS28 are (bilaterally): proximal interphalangeal joints (10 joints), metacarpophalangeal joints (10), wrists (2), elbows (2), shoulders (2) and knees (2). When looking at these joints, both the number of joints with tenderness upon touching (TEN28) and swelling (SW28) are counted. In addition, the erythrocyte sedimentation rate (ESR) is measured. Also, the patient makes a subjective assessment (SA) of disease activity during the preceding 7 days on a scale between 0 and 100, where 0 is "no activity" and 100 is "highest activity possible". With these parameters, DAS28 is calculated as:[24] 
From this, the disease activity of the patient can be classified as follows:[24]
Current
DAS28 |
DAS28 difference from initial value |
|
> 1.2 |
> 0.6 but ≤ 1.2 |
≤ 0.6 |
|
≤ 3.2 |
Inactive |
Good improvement |
Moderate improvement |
No improvement |
|
> 3.2 but ≤ 5.1 |
Moderate |
Moderate improvement |
Moderate improvement |
No improvement |
|
> 5.1 |
Very active |
Moderate improvement |
No improvement |
No improvement |
[edit] Pathophysiology and causes
Rheumatoid arthritis is a form of autoimmunity, the causes of which are still incompletely known. It is a systemic (whole body) disorder principally affecting synovial tissues.
The key pieces of evidence relating to pathogenesis are:
1. A genetic link with HLA-DR4 and related allotypes of MHC Class II and the T cell-associated protein PTPN22.
2. A undeniable link with cigarette smoking and the pathogenesis of rheumatoid vasculitis, a typical feature of this condition.[25]
3. A remarkable deceleration of disease progression in many cases by blockade of the cytokine TNF (alpha).
4. A similar dramatic response in many cases to depletion of B lymphocytes, but no comparable response to depletion of T lymphocytes.
5. A more or less random pattern of whether and when predisposed individuals are affected.
6. The presence of autoantibodies to IgGFc, known as rheumatoid factors (RF), and antibodies to citrullinated peptides (ACPA).
These data suggest that the disease involves abnormal B cell–T cell interaction, with presentation of antigens by B cells to T cells via HLA-DR eliciting T cell help and consequent production of RF and ACPA. Inflammation is then driven either by B cell or T cell products stimulating release of TNF and other cytokines. The process may be facilitated by an effect of smoking on citrullination but the stochastic (random) epidemiology suggests that the rate limiting step in genesis of disease in predisposed individuals may be an inherent stochastic process within the immune response such as immunoglobulin or T cell receptor gene recombination and mutation. (See entry under autoimmunity for general mechanisms.)
If TNF release is stimulated by B cell products in the form of RF or ACPA -containing immune complexes, through activation of immunoglobulin Fc receptors, then RA can be seen as a form of Type III hypersensitivity.[26] [27] If TNF release is stimulated by T cell products such as interleukin-17 it might be considered closer to type IV hypersensitivity although this terminology may be getting somewhat dated and unhelpful.[28] The debate on the relative roles of immune complexes and T cell products in inflammation in RA has continued for 30 years. There is little doubt that both B and T cells are essential to the disease. However, there is good evidence for neither cell being necessary at the site of inflammation. This tends to favour immune complexes (based on antibody synthesised elsewhere) as the initiators, even if not the sole perpetuators of inflammation. Moreover, work by Thurlings and others in Paul-Peter Tak's group and also by Arthur Kavanagh's group suggest that if any immune cells are relevant locally they are the plasma cells, which derive from B cells and produce in bulk the antibodies selected at the B cell stage.[citation needed]
Although TNF appears to be the dominant, other cytokines (chemical mediators) are likely to be involved in inflammation in RA. Blockade of TNF does not benefit all patients or all tissues (lung disease and nodules may get worse). Blockade of IL-1, IL-15 and IL-6 also have beneficial effects and IL-17 may be important. Constitutional symptoms such as fever, malaise, loss of appetite and weight loss are also caused by cytokines released in to the blood stream.
As with most autoimmune diseases, it is important to distinguish between the cause(s) that trigger the process, and those that may permit it to persist and progress.
[edit] Possible infectious triggers
It has long been suspected that certain infections could be triggers for this disease. The "mistaken identity" theory suggests that an infection triggers an immune response, leaving behind antibodies that should be specific to that organism. The antibodies are not sufficiently specific, though, and set off an immune attack against part of the host. Because the normal host molecule "looks like" a molecule on the offending organism that triggered the initial immune reaction—this phenomenon is called molecular mimicry. Some infectious organisms suspected of triggering rheumatoid arthritis include Mycoplasma, Erysipelothrix, parvovirus B19 and rubella, but these associations have never been supported in epidemiological studies. Nor has convincing evidence been presented for other types of triggers such as food allergies.
Epidemiological studies have confirmed a potential association between RA and two herpesvirus infections: Epstein-Barr virus (EBV) and Human Herpes Virus 6 (HHV-6).[29] Individuals with RA are more likely to exhibit an abnormal immune response to the Epstein-Barr virus.[30] [31] The allele HLA-DRB1*0404 is associated with low frequencies of T cells specific for the EBV glycoprotein 110 and predisposes one to develop RA.[32]
[edit] Psychological factors
There is no evidence that physical and emotional effects or stress could be a trigger for the disease. The many negative findings suggest that either the trigger varies, or that it might in fact be a chance event inherent with the immune response, as suggested by Edwards et al.[33]
[edit] Continued abnormal immune response
The factors that allow an abnormal immune response, once initiated, to become permanent and chronic, are becoming more clearly understood. The genetic association with HLA-DR4, as well as the newly discovered associations with the gene PTPN22 and with two additional genes,[34] all implicate altered thresholds in regulation of the adaptive immune response. It has also become clear from recent studies that these genetic factors may interact with the most clearly defined environmental risk factor for rheumatoid arthritis, namely cigarette smoking[35][36] Other environmental factors also appear to modulate the risk of acquiring RA, and hormonal factors in the individual may explain some features of the disease, such as the higher occurrence in women, the not-infrequent onset after child-birth, and the (slight) modulation of disease risk by hormonal medications. Exactly how altered regulatory thresholds allow the triggering of a specific autoimmune response remains uncertain. However, one possibility is that negative feedback mechanisms that normally maintain tolerance of self are overtaken by aberrant positive feedback mechanisms for certain antigens such as IgG Fc (bound by RF) and citrullinated fibrinogen (bound by ACPA) (see entry on autoimmunity).
Once the abnormal immune response has become established (which may take several years before any symptoms occur), plasma cells derived from B lymphocytes produce rheumatoid factors and ACPA of the IgG and IgM classes in large quantities. These are not deposited in the way that they are in systemic lupus. Rather, they appear to activate macrophages through Fc receptor and perhaps complement binding. This can contribute to inflammation of the synovium, in terms of edema, vasodilation and infiltration by activated T-cells (mainly CD4 in nodular aggregates and CD8 in diffuse infiltrates). Synovial macrophages and dendritic cells further function as antigen presenting cells by expressing MHC class II molecules, leading to an established local immune reaction in the tissue. The disease progresses in concert with formation of granulation tissue at the edges of the synovial lining (pannus) with extensive angiogenesis and production of enzymes that cause tissue damage. Modern pharmacological treatments of RA target these mediators. Once the inflammatory reaction is established, the synovium thickens, the cartilage and the underlying bone begins to disintegrate and evidence of joint destruction accrues.
[edit] Treatment
There is no known cure for rheumatoid arthritis, but many different types of treatment can alleviate symptoms and/or modify the disease process. Recommendations of the American College of Rheumatology (ACR), published in 2008, followed a trend in supporting earlier, more aggressive treatment of RA, and reflected heightened expectations of treatment effectiveness, including remission or substantial alleviation of symptoms for a rising percentage of patients.[37]
The goal of treatment is twofold: alleviating the current symptoms, and preventing the future destruction of the joints with the resulting handicap if the disease is left unchecked. These two goals may not always coincide: while pain relievers may achieve the first goal, they do not have any impact on the long-term consequences. For these reasons, the ACR recommends that RA should generally be treated with at least one specific anti-rheumatic medication, also named DMARD (see below), to which other medications may be added depending on how long a person has had RA, how active the disease is, and prognostic factors (such as X-ray evidence of bone erosion; elevation of blood factors such as Rheumatoid factor, anti-cyclic citrullinated peptide, C-reactive protein, and erythrocyte sedimentation rate; age and gender; physical functioning; and smoking, for example).[37]
Cortisone therapy has offered relief in the past, but its long-term effects have been deemed undesirable.[38] However, cortisone injections can be valuable adjuncts to a long-term treatment plan, and using low dosages of daily cortisone (e.g., prednisone or prednisolone, 5–7.5 mg daily) can also have an important benefit if added to a proper specific anti-rheumatic treatment.[citation needed]
Pharmacological treatment of RA can be divided into disease-modifying antirheumatic drugs (DMARDs), anti-inflammatory agents and analgesics.[39][40] Treatment also includes rest and physical activity.
[edit] Disease modifying anti-rheumatic drugs (DMARDs)
The term Disease-modifying anti-rheumatic drug (DMARD) originally meant a drug that affects biological measures such as ESR and haemoglobin and autoantibody levels, but is now usually used to mean a drug that reduces the rate of damage to bone and cartilage. DMARDs have been found both to produce durable symptomatic remissions and to delay or halt progression. This is important as such damage is usually irreversible. Anti-inflammatories and analgesics improve pain and stiffness but do not prevent joint damage or slow the disease progression.
There is an increasing recognition among rheumatologists that permanent damage to the joints occurs at a very early stage in the disease. In the past it was common to start with just an anti-inflammatory drug, and assess progression clinically and using X-rays. If there was evidence that joint damage was starting to occur then a more potent DMARD would be prescribed. Ultrasound and MRI are more sensitive methods of imaging the joints and have demonstrated that joint damage occurs much earlier and in more sufferers than was previously thought. People with normal X-rays will often have erosions detectable by ultrasound that X ray could not demonstrate. The aim now is to treat before damage occurs.
There may be other reasons why starting DMARDs early is beneficial as well as prevention of structural joint damage. From the earliest stages of the disease, the joints are infiltrated by cells of the immune system that signal to one another in ways that may involve a variety of positive feedback loops (it has long been observed that a single corticosteroid injection may abort synovitis in a particular joint for long periods). Interrupting this process as early as possible with an effective DMARD (such as methotrexate) appears to improve the outcome from the RA for years afterwards. Delaying therapy for as little as a few months after the onset of symptoms can result in worse outcomes in the long term. There is therefore considerable interest in establishing the most effective therapy with early arthritis, when they are most responsive to therapy and have the most to gain.[41]
Disease-modifying anti-rheumatic drugs have been used in the treatment of rheumatic arthritis for a long time now. Over 90% of rheumatologists now use combination therapy of multiple disease modifying drugs for rheumatoid arthritis as it has become apparent that using combination of these drugs does not increase their relative toxicity profiles.[42] Common combinations of DMARDs include methotrexate – hydroxychloroquine, methotrexate – sulfasalazine, sulfasalazine – hydroxychloroquine, and methotrexate – hydroxychloroquine – sulfasalazine.
In order to be effective, disease-modifying anti-rheumatic drugs must be administered before the deformities appear or the erosive disease occurs. Usually, Rheumatologists do not wait for the fulfillment of the criteria for classification of RA as published by the American College of Rheumatology (ACR) and start treatment with this type of drugs if the pain and synovitis persist and the function is compromised.[43]
[edit] Traditional small molecular mass drugs
Chemically synthesised DMARDs:
Cytotoxic drugs:
The most important and most common adverse events relate to liver and bone marrow toxicity (MTX, SSZ, leflunomide, azathioprine, gold compounds, D-penicillamine), renal toxicity (cyclosporine A, parenteral gold salts, D-penicillamine), pneumonitis (MTX), allergic skin reactions (gold compounds, SSZ), autoimmunity (D-penicillamine, SSZ, minocycline) and infections (azathioprine, cyclosporine A).
Hydroxychloroquine may cause ocular toxicity, although this is rare, and because hydroxychloroquine does not affect the bone marrow or liver it is often considered to be the DMARD with the least toxicity. Unfortunately hydroxychloroquine is not very potent, and is usually insufficient to control symptoms on its own.[citation needed]
Methotrexate is considered by many rheumatologists to be the most important and useful DMARD, largely because of lower drop-out rates for reasons of toxicity. Nevertheless, methotrexate is often considered as a very 'toxic' drug. This reputation is not entirely justified, and at times can result in people being denied the most effective treatment for their arthritis. Although methotrexate does have the potential to suppress bone marrow or cause hepatitis, these effects can be monitored using regular blood tests, and the drug withdrawn at an early stage if the tests are abnormal before any serious harm is done (typically the blood tests return to normal after stopping the drug). In clinical trials, where one of a range of different DMARDs were used, people who were prescribed methotrexate stayed on their medication the longest (the others stopped because of either side-effects or failure of the drug to control the arthritis).[citation needed] Methotrexate is often preferred by rheumatologists because if it does not control arthritis on its own then it works well in combination with many other drugs, especially the biological agents. Other DMARDs may not be as effective or as safe in combination with biological agents.[citation needed]
Sulphasalazine : Although it appears to be a highly efficient drug in the treatment of rheumatoid arthritis, sulphasalazine may cause side effects that can range in severity from mild to serious. Mild side effects that may arise from treatment with sulphasalazine include nausea and skin rash. Generally, nausea that appears as a result of treatment with this DMARD occurs in the first days of treatment and then it tends to diminish to disappearance. To avoid nausea, specialists recommend starting with low doses and then gradually increasing them until the usual dosage is achieved. Skin rash has been reported in nearly 5% of the patients and it may present pruritus. Rare side effects include Stevens–Johnson syndrome and reduced fertility due to reversible oligospermia. Severe side effects that can appear from therapy with sulphasalazine, though rare, are aplastic anemia and neutropenia which may result in the death of the patient. The latter is estimated to have occurred in approximately 2% of the patients but death and further complications were avoided by removing the drug from the patient's therapy. Also, according to WHO, there have been approximately 700 of patients in whom this medicine caused blood dyscrasis. Leukopenia has also been reported in therapies with sulphasalazine, but in very rare cases.
Anti-malarials such as chloroquine and hydroxychloroquine have been used to treat rheumatoid arthritis. It has been pointed out, through clinical studies, that chloroquine has a higher toxicity compared to hydroxychloroquine. Although hydroxyxhloroquine appears to be more efficient in treating rheumatoid arthritis than placebo, it is also inferior to sulphasalazine, especially in what concerns preventing the joint damage that is caused by the disease. As most drugs, anti-malarials may also produce side effects. Mild side effects from hydroxychloroquine include nausea and skin rash. More serious, bone marrow suppression may occur, though rare. Also, aplastic anemia and agranulocytosis can develop as a result of anti-malarial therapy and may potentially cause the death of the patient. A much more worrisome side effect from treatment with anti-malarials is the damage that these drugs seem to be causing to the cornea and retina. Recent studies have however shown that if the dosage of hydroxychloroquine given to the patients does not exceed 6.5 mg/kg, the risks of developing ocular complications are minimal.[44]
Gold compounds are also options in treating this type of disease. Specialists agree that injectable gold is much more effective in the treatment of rheumatoid arthritis than auranofin. Yet, this type of drug has been shown to be more efficient than placebo and even though its level of toxicity is quite low, auranofin seems to be causing more side effects than any other type of DMARD. Auranofin is therefore not considered efficient in the treatment of rheumatoid arthritis because of its poor results and because it is intolerable for most patients. Sodium aurothiomalate (Myocrisin) on the other hand is another type of gold compound that is injected and which appears to be as efficient as sulphasalazine, d-penicillamine and methotrexate. Given that there is not enough proof that gold compounds are indeed efficient in preventing the progression of erosions and the high toxicity of these drugs, they are usually not included in the treatment plan for rheumatoid arthritis.
A Cochrane systematic review has determined that Abatacept was an effective treatment for rheumatoid arthritis. Against placebo, it was found to increase mobility and make patients twice as likely to achieve a 50% improvement in symptoms.[45] However Cochrane has called for more studies to be conducted, given the lack of evidence to distinguish between the biologics available for rheumatoid arthritis.[46]
[edit] Biological agents
Biological agents (biologics) include:
[edit] Anti-inflammatory agents and analgesics
Anti-inflammatory agents include:
Analgesics include:
Historic treatments for RA have also included: rest, ice, compression and elevation, acupuncture, apple diet, nutmeg, some light exercise every now and then, nettles, bee venom, copper bracelets, rhubarb diet, extractions of teeth, fasting, honey, vitamins, insulin, magnets, and electroconvulsive therapy (ECT).[51] Most of these have either had no effect at all, or their effects have been modest and transient, while not being generalizable.[citation needed]
NSAIDs used in the treatment of RA include ibuprofen, naproxen, meloxicam, etodolac, nabumetone, sulindac, tolementin, choline magnesium salicylate, diclofenac, diflusinal, indomethicin, Ketoprofen, Oxaprozin, and piroxicam.
Cortisone therapy became a controversial medical solution because even though it can provide great relief, there are some questions as to the usefulness of the procedure over a long period of time.[52]
[edit] Surgery
In early phases of the disease, an arthroscopic or open synovectomy may be performed. It consists of the removal of the inflammed synovia and prevents a quick destruction of the affected joints. In older patients, the yttrium synovectomy may be performed. It is successful in approximately half of patients. The surgery is mostly done on knee, elbow, shoulder, ankle or tarsal joints. It has to be performed before the destruction of the cartilage.[citation needed] Severely affected joints may require joint replacement surgery, such as knee replacement. Postoperatively, physiotherapy is always necessary.
[edit] Other therapies
Other therapies are weight loss, orthoses, occupational therapy, podiatry, physiotherapy, immunoadsorption therapy, joint injections, and special tools to improve hand movements (e.g., special tin-openers). Regular exercise is important for maintaining joint mobility and making the joint muscles stronger. A Cochrane Review of studies determined that exercise programs designed to improve strength and stamina were safe and led to moderate benefits for RA sufferers.[53]
Ayurveda, mostly in southern India, is another source of treatment, and while it is popular in India there are no studies to show that it benefits patients with RA[citation needed].
One survey in the United Kingdom between 1998 and 2002 found that arthritis, in its various forms, was among the five most common reasons for the medicinal use of cannabis.[54]
The Prosorba column blood filtering device (removing IgG) was approved by the FDA in 1999 for treatment of RA[55] However it was discontinued at the end of 2006.[56]
The effectiveness of treating RA with acupuncture is inconclusive, and "more rigorous research seems to be warranted" according to one study.[57]
One study of 873 patients with RA found that those who drank some alcohol (none drank more than 10 units of alcohol a week) had reduced severity of symptoms compared to those who drank no alcohol.[58][59] However a spokeswoman for the Arthritis Research UK (who co-funded the study) warned that some RA treatments, like methotrexate, could damage the liver when taken with large amounts of alcohol.[59]
[edit] Prognosis
The course of the disease varies greatly. Some people have mild short-term symptoms, but in most the disease is progressive for life. Around 20%–30% will have subcutaneous nodules (known as rheumatoid nodules); this is associated with a poor prognosis.
[edit] Prognostic factors
Poor prognostic factors include persistent synovitis, early erosive disease,extra-articular findings (including subcutaneous rheumatoid nodules), positive serum RF findings, positive serum anti-CCP autoantibodies, carriership of HLA-DR4 "Shared Epitope" alleles, family history of RA, poor functional status, socioeconomic factors, elevated acute phase response (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), and increased clinical severity.
[edit] Mortality
Estimates of the life-shortening effect of RA vary; most sources cite a lifespan reduction of 5 to 10 years. According to the UK's National Rheumatoid Arthritis Society, "Young age at onset, long disease duration, the concurrent presence of other health problems (called co-morbidity), and characteristics of severe RA—such as poor functional ability or overall health status, a lot of joint damage on x-rays, the need for hospitalisation or involvement of organs other than the joints—have been shown to associate with higher mortality".[60] Positive responses to treatment may indicate a better prognosis. A 2005 study by the Mayo Clinic noted that RA sufferers suffer a doubled risk of heart disease,[61] independent of other risk factors such as diabetes, alcohol abuse, and elevated cholesterol, blood pressure and body mass index. The mechanism by which RA causes this increased risk remains unknown; the presence of chronic inflammation has been proposed as a contributing factor.[62]
[edit] Epidemiology

Disability-adjusted life year for rheumatoid arthritis per 100,000 inhabitants in 2004.
[63]
no data
less than 40
40–50
50–60
60–70
70–80
80–90
90–100
100–110
110–120
120–130
130–140
more than 140
The incidence of RA is in the region of 3 cases per 10,000 population per annum. Onset is uncommon under the age of 15 and from then on the incidence rises with age until the age of 80. The prevalence rate is 1%, with women affected three to five times as often as men. It is up to three times more common in smokers than non-smokers, particularly in men, heavy smokers, and those who are rheumatoid factor positive.[36] A study in 2010 found that those who drank modest amounts of alcohol regularly were four times less likely to get rheumatoid arthritis than those who never drank.[58][64] Some Native American groups have higher prevalence rates (5–6%) and people from the Caribbean region have lower prevalence rates. First-degree relatives prevalence rate is 2–3% and disease genetic concordance in monozygotic twins is approximately 15–20%.[65][66]
It is strongly associated with the inherited tissue type Major histocompatibility complex (MHC) antigen HLA-DR4 (most specifically DR0401 and 0404)—hence family history is an important risk factor.[citation needed]
The risk of first developing the disease (the disease incidence) appears to be greatest for women between 40 and 50 years of age, and for men somewhat later.[67] RA is a chronic disease, and although rarely, a spontaneous remission may occur, the natural course is almost invariably one of persistent symptoms, waxing and waning in intensity, and a progressive deterioration of joint structures leading to deformations and disability.
[edit] History
The first known traces of arthritis date back at least as far as 4500 BC. A text dated 123 AD first describes symptoms very similar to rheumatoid arthritis. It was noted in skeletal remains of Native Americans found in Tennessee.[68] In the Old World the disease is vanishingly rare before the 1600s.[69] and on this basis investigators believe it spread across the Atlantic during the Age of Exploration. In 1859 the disease acquired its current name.
An anomaly has been noticed from investigation of Precolumbian bones. The bones from the Tennessee site show no signs of tuberculosis even though it was prevalent at the time throughout the Americas.[70] Jim Mobley, at Pfizer, has discovered a historical pattern of epidemics of tuberculosis followed by a surge in the number of rheumatoid arthritis cases a few generations later.[71] Mobley attributes the spikes in arthritis to selective pressure caused by tuberculosis. A hypervigilant immune system is protective against tuberculosis at the cost of an increased risk of autoimmune disease.
The art of Peter Paul Rubens may possibly depict the effects of rheumatoid arthritis. In his later paintings, his rendered hands show, in the opinion of some physicians, increasing deformity consistent with the symptoms of the disease.[72][73] Rheumatoid arthritis appears to some to have been depicted in 16th century paintings.[74] However, it is generally recognised in art historical circles that the painting of hands in the sixteenth and seventeenth century followed certain stylised conventions, most clearly seen in the Mannerist movement. It was conventional, for instance to show the upheld right hand of Christ in what now appears a deformed posture. These conventions are easily misinterpreted as portrayals of disease. They are much too widespread for this to be plausible.
The first recognized description of rheumatoid arthritis was in 1800 by the French physician Dr Augustin Jacob Landré-Beauvais (1772–1840) who was based in the famed Salpêtrière Hospital in Paris.[2] The name "rheumatoid arthritis" itself was coined in 1859 by British rheumatologist Dr Alfred Baring Garrod.[75]
[edit] Notable cases
-
Christiaan Barnard, the first surgeon to perform a human-to-human heart transplant had to retire owing to the condition. He also wrote a book on living with arthritis.
-
Billy Bowden, international cricket umpire who had to retire from active play because of rheumatoid arthritis.
-
James Coburn claimed to have healed the condition using pills containing a sulfur-containing compound on his return to acting.
-
Raoul Dufy, French artist (1877–1953), continued to paint despite RA and was one of the first patients ever treated with cortisone, in a Boston hospital.
-
Jamie Farr, American actor, famous for his role as Max Klinger on the 1970s television series M*A*S*H.
-
Melvin Franklin, bass singer of the Temptations. He treated RA with cortisone shots so he could perform.
-
Dorothy Hodgkin, Nobel prize winning scientist, developed severe deforming rheumatoid arthritis at age 28. In spite of this she continued her career and developed X-ray crystallography, which underpins much of the information known about rheumatoid arthritis. She also discovered the structure of insulin and enabled the discovery of the genetic code.
-
Matt Iseman, licensed physician and professional comedian, and host of Style Network's Clean House.
-
Sandy Koufax, an American Hall-of-Fame baseball pitcher who played from 1955 to 1966 for the Los Angeles Dodgers.
-
Erik Lindbergh, aviator and member of the X-Prize administration. Erik has been a spokesman for the arthritis drug Enbrel, as a result of his success with the treatment.[76]
-
Bob Mortimer British comedian and actor.[77]
-
Auguste Renoir, impressionist painter, whose later 'softer' style might have reflected in some way his severe disability.
-
Kathleen Turner and Aida Turturro have worked to raise public awareness of the condition[78]
[edit] See also
| Author: | Bling King |
| Published: | Sep 24th 2011 |
| Modified: | Jan 10th 2012 |